We use cookies to enhance your browsing experience. If you are OK with this please click Opt-In. You can always remove your permission by clicking Opt-Out on the Privacy Policy page.
Diamond & cBN
Diamond's hardness is the source of its name (from the ancient Greek adámas “unbreakable”).
Diamonds were first mined in India, approximately 3,000 years ago, in alluvial deposits, along several rivers.
Diamond is the hardest material known having a Vickers hardness in the range of 70–150 GPa. It also has a high thermal conductivity and electrical insulating properties. Most diamond used in industrial applications is synthetic i.e. produced by converting graphite to diamond, at high pressure and high temperature.
Cubic boron nitride (CBN) has a Vickers hardness of 48GPa and is insoluble in iron, nickel, and related alloys at high temperatures. Although it is less hard than diamond it is often used in applications where diamond suffers from "chemical wear".
Materials consisting of elements from the B-C-N triangle are amongst the hardest materials, they include diamond and cubic boron nitride (cBN).
Antoine Lavoisier
In 1772, Antoine Lavoisier used a lens to concentrate the rays of the sun onto a diamond. In an atmosphere of oxygen, the only product of the combustion was carbon dioxide, proving that diamond is an allotrope of carbon.
As the lightest element with 4 valence electrons, carbon has the unique ability to form a variety of stable covalent bonds with many elements, including itself.
Bohr & electron cloud models of carbon

Diamond & Cubic Boron Nitride morphology
Graphite
Tetrahedron
Diamond
Carbon atoms in graphite are arranged in layers. Each carbon atom is bonded to three other carbon atoms at the corners of regular hexagons with a 120° C-C-C bond angle. These planar arrangements extend in 2D to form horizontal, hexagonal arrays. The distance between two layers is much greater (0.335 nm) than the distance between carbon atoms within each layer (0.142nm). Graphite can be used as a lubricant because the layers slide over one another. The structure of graphite allows electrons to move easily within the planes allowing the conduction of electricity and heat.
In diamond, carbon atoms are arranged tetrahedrally, where each carbon atom is attached to four other carbon atoms 0.154 nm away with a C-C-C bond angle of 109.5°. It is a strong, rigid 3D structure which accounts for diamond's hardness, strength, durability, high melting point and high resistance to compression.
Diamond

cBN
{100} 4 point
{110} 2 point
{111} 3 point
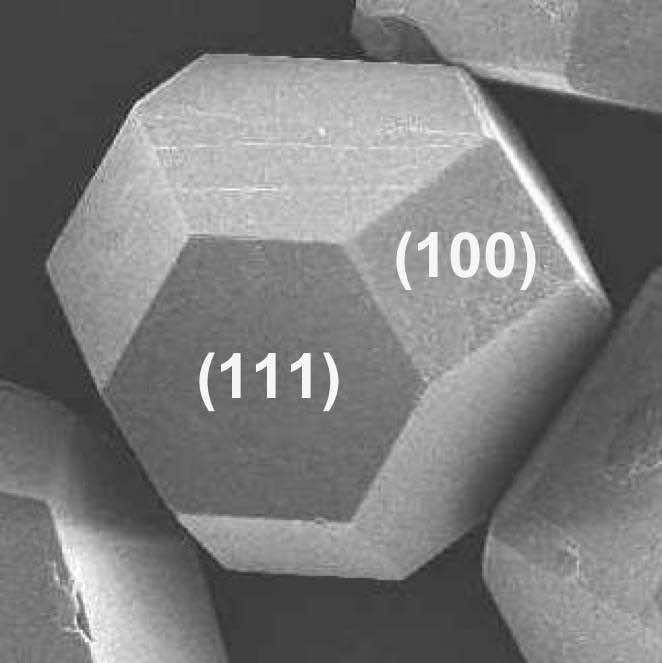
The diagram shows the morphology evolution from cube (1) to octahedron (5):
- Cube.
- Corner-truncated Cube (triangular faces on cube corners).
- Cuboctahedron (triangular faces at maximum size).
- Corner-truncated Octahedron (triangular faces meet to form a hexagon).
- Octahedron.
In practice, diamond can range from cubooctahedral to partially grown, irregular and fragmented crystals.
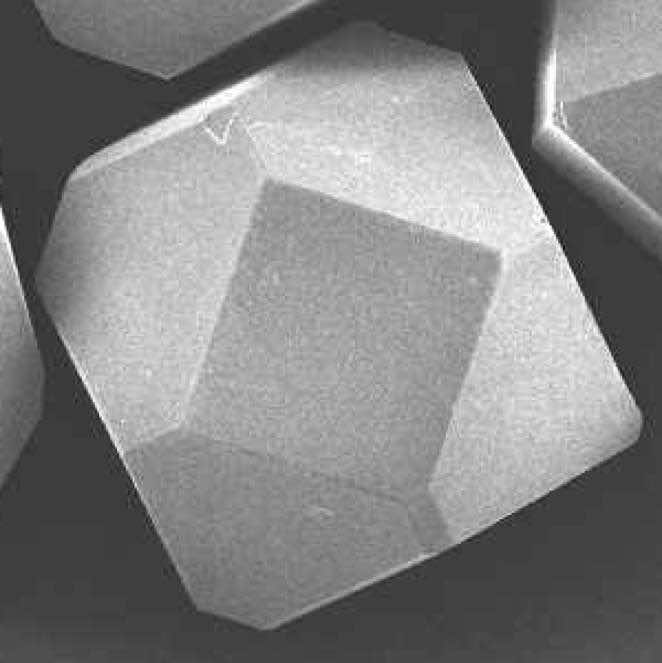
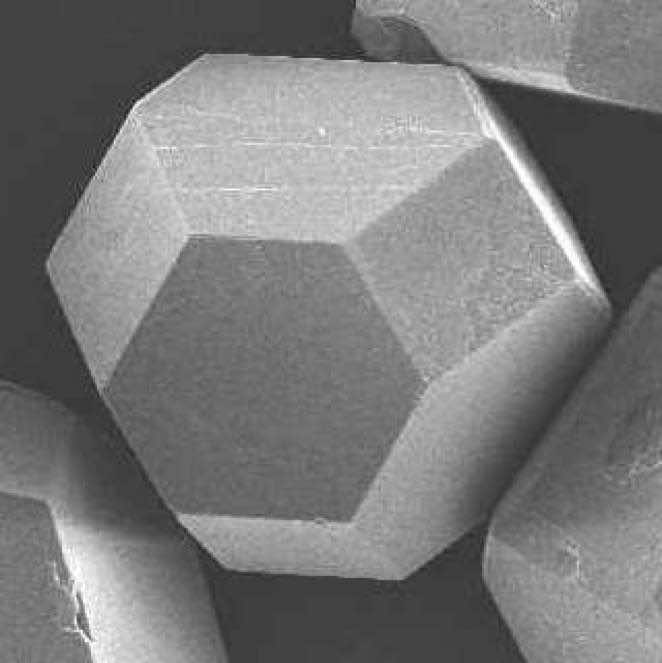
The black and white photographs show two common synthetic diamond grit shapes.

All five morphologies possess the same underlying cubic structure.
cBN


Amber coloured cBN crystals

Different products can be selected by cBN morphology.
Diamond formation
The formation of diamond requires specific conditions; exposure of carbon bearing materials to high pressure (45,000 to 60,000 bar) and high temperature (900 to 1,300 °C).
These conditions occur in 3 places on Earth:
- in the mantle below the continental plates,
- at the site of a meteor strike and
- in high pressure, high temperature presses (HPHT).

Diamond in reaction mass



When diamond is grown in high-pressure high-temperature systems, using solvent-catalysts (such as nickel, cobalt or iron) to reduce the kinetic barrier and act as transport media for dissolved carbon, metallic inclusions are formed which affect the properties of the diamond. Synthetic diamond typically has a yellow hue because of nitrogen incorporation into the diamond lattice from the atmosphere and growth materials.
Nitrogen can comprise up to 1% of a diamond's mass:
Type I - contains nitrogen, typically 100 to 3,000 ppm
Type Ia - contains aggregated nitrogen
Type Ib - contains single substitutional nitrogen
Type II - very low nitrogen concentration, typically <5 ppm
Type IIa - nitrogen is the major impurity
Type IIb - boron is a substitutional impurity
Chemical vapour deposition (CVD)
Chemical vapour deposition is a process by which diamond is grown from a hydrocarbon gas mixture. The advantages of CVD are the ability to grow diamond over large surfaces, on various substrates, with control over the diamond’s properties. Unlike high-pressure high-temperature systems, the process does not require extreme pressures.
CVD diamond growth involves substrate preparation, optimization of the substrate temperature, feeding varying amounts of gases into a chamber and exciting them. These gases include a carbon source, typically methane, as well as hydrogen, which is essential, because it etches non-diamond carbon. In the growth chamber, gases are ionized into chemically active radicals, using microwave power or other power sources.
CVD diamond is classified by its grain size: < 10 nm, < 50 nm, < 500 μm and single crystal (Type IIa). By controlling the impurities and grain boundaries free standing polycrystalline diamond wafers can also be produced.

